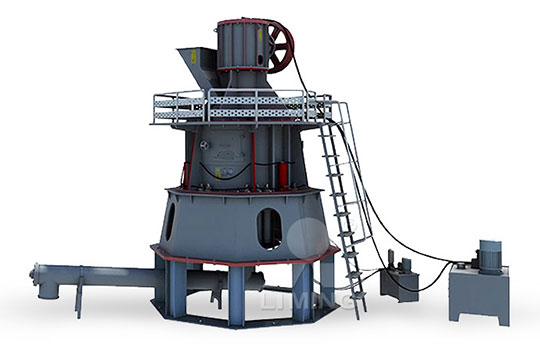
How to calculate the calcium carbonate grinding system
.jpg)
CALCIUM CARBONATE (GCC) Hosokawa Alpine
The Hosokawa Alpine ATR system in a circuit with a highperformance air classifier is the ultimate energysaving solution for the generation of ultrafine GCC fillers in the fineness range of d 97 = 25 – 8 µm Compared to ball milling systems, the system impresses with significantly lower calcium carbonate (PCC) with a relatively low bulk density, Sample B is a granular calcium carbonate with a much higher bulk density The diagram on page 3 shows a schematic Conveying and Feeding of Calcium Carbonate in Plastics Dry grinding of calcium carbonate using pendulum mills is an essential technique for obtaining highquality powders tailored to the specific requirements of modern industriesGrinding calcium carbonate: techniques, challenges In this study, we analysed foodgrade calcium carbonates from different manufacturers, evaluating particle size and specific surface area The particle size distribution was analysed using the MalvernPanalytical Mastersizer 3000 Calcium carbonate: evaluating particle size and surface

Calcium Carbonate (GCC) Hosokawa Alpine
In the production of ultrafine calcium carbonate additives, various aspects must be considered when selecting and deciding among available types of processing systems: Fineness range, annual output (uncoated and coated GCC), dry or 2021年12月20日 Prater’s air classifiers are used within systems designed for calcium carbonate manufacturing Processing the powdered calcium carbonate by separating differently sized particles via centrifugal force, works within Calcium Carbonate Manufacturing Process and 2021年12月31日 Grinding aid chemicals which are used in the grinding of calcium carbonate (CaCO3) to prevent agglomeration are chemisorbed on the surfaces of particles, and the compatibility of them with(PDF) Effects of Grinding Aids Used in Grinding 2023年8月1日 Particle size, energy consumption, and grinding media coating were analyzed Powder flowability and surface area were evaluated by different methods These grinding aids Effective role of grinding aids in the dry grinding performance of

Characteristics of the Treated Ground Calcium Carbonate Powder
This study examined the surface properties, fluidity, flowability and floodability of untreated ground calcium carbonate (GCC) powder and treated GCC powder with stearic acid (SA) using a dry H 2 CO 3, the rather weak carbonic acid, is important in controlling the solubility of carbonate minerals in most natural environmentsFor example, the decay of organic matter in a soil may produce CO 2 in large amounts The gas produced is readily soluble in water and a proportion combines with water to yield carbonic acid ()An increase in activity of the carbonic acid in Calcium carbonate and the carbonic acid system SpringerLink2020年7月30日 In this study, to determine the calcium carbonate availability in eggshells waste and factors those affect its extraction The parameters like temperature, the size of the eggshell powder and the (PDF) Calcium Carbonate Synthesis, Optimization assumption that passive scales were made up of iron hydroxide codeposited with calcium carbonate (we now know that it’s actually ferrous carbonate that forms) The LSI is based on the algebraic manipulation of the active ion product for calcium carbonate and that of the dissociation of the bicarbonate ion: 2+αCa 2 αCO 3 = Ksp (1) +αCO 3A NOVEL CALCIUM CARBONATE SCALING MODEL FOR
.jpg)
Carbonate Minerals and the CO2Carbonic Acid System
2018年1月1日 CO 2 hydrolyzes in water, making the bicarbonate and carbonate anions (discussed in more detail in section “CO 2Carbonic AcidCarbonate System and Seawater”) that react with divalent and monovalent metals 3 Among the divalent cations, calcium combined with the carbonate anion makes a solid of low solubility2023年6月25日 Crushing: The calcium carbonate stones just mined from the quarry are relatively large, and they need to be crushed by a jaw crusher and a hammer crusher in turn to the feed fineness (10mm20mm) that can enter the mill Grinding: Use a bucket elevator to send the crushed small pieces of calcium carbonate to the silo, then use a vibrating feeder to send them Guide to Calcium Carbonate Grinding: Mills, Tips, and Uses2020年12月28日 The calcium carbonate (CaCO3) precipitation potential (CCPP) can predict the potential for corrosion and lime scaling in drinking water systems CCPP can be calculated by different standards, but none of these consider all of the conditions in drinking water systems where temperatures can reach 100 °C and the water exchanges CO2 with the atmosphere Procedure for Calculating the Calcium Carbonate Precipitation 2002年3月11日 Grinding tests were carried out to determine the influence of optimum dispersant dosage on grinding mill performance All grinding tests were carried out in a Svedala 75 kW SAM (Sala Agitated Mill) mill; see Fig 1The SAM mill was developed for grinding middlings concentrates in mineral beneficiation processes as well as for fine or ultrafine grinding of A new method for determining the optimum dispersant concentration
.jpg)
CARBONATE EQUILIBRIA UC Davis
O system Calcium carbonate in water with a fixed partial pressure of carbon dioxide For the case of a fixed partial pressure of carbon dioxide and calcium carbonate dissolved in the aqueous phase one more equation is need to describe the system This is the solubility product of calcium carbonate: = K ( CaCO ) ( Ca ) ( CO ) o CaCO 32019年7月22日 Recommend Calcium Carbonate Grinding Mill Capacity: 0430 T/H Feeding size: ≤20mm Output Size: 1503000mesh Calcium Carbonate Grinding Mill is the equipment specializing in producing fine and superfine calcium carbonate powderHow to make the calcium carbonate powder?2018年6月4日 4 Calculating Alkalinity as CaCO₃ To calculate alkalinity as calcium carbonate, use this formula to calculate the alkalinity in concentration of calcium carbonate (CaCO 3) Insert the values into the formula to determine the alkalinity as CaCO 3: Alk(mg/L as CaCO 3)=(50044xBxC a xCF)÷V s Inserting the example values, the equation becomes:How To Calculate Alkalinity As Concentration Of CaCO32015年1月27日 To find the molar mass of the metal carbonate, we took the mass of the sample used (the metal carbonate) and divided it by moles of $\ce{CO2}$ released, which was just calculated The rest here on out was a little algebra What is the reasoning behind dividing the mass of the sample by released $\ce{CO2}$?How to find the molar mass of an unknown metal carbonate

Calcium Carbonate (GCC) Hosokawa Alpine
In the production of ultrafine calcium carbonate additives, various aspects must be considered when selecting and deciding among available types of processing systems: Fineness range, annual output (uncoated and coated GCC), dry or 2016年11月6日 You take the atomic mass of calcium over the atomic mass of #CaCO3# and multiply it by 100 to get the percentage The mass of #Ca# is 40078 g; the mass of #C# is 1201 g, and the mass of #3O# is 47997 g (#"15999 g" xx 3#)Add all the masses together to get a total mass of 100085 gHow do you calculate the percentage of calcium in calcium carbonate As the CO 2 content has an influence on pH, we can conclude that, for a given mineralisation (fixed CaH and Malk), there will be a pH value for the equilibrium between this water and the calcium carbonate: this value is termed the Langelier pH or saturation pH hence the designation "pH S" (the corresponding CO 2 content then being know as the balancing CO 2, a concept water treatment the calciumcarbonate balance Degremont®Effects of Grinding Aids Used in Grinding Calcium Carbonate (CaCO 3 ) Filler on the Properties of WaterBased Interior Paints Orkun Ersoy 1, * , Dilek Güler 2 and Murat Rençberoglu˘ 21,* , Dilek Güler 2 ResearchGate
.jpg)
How to calculate the final concentration of calcium ions from
2015年6月3日 The maximum amount of $\ce{CaCO3}$ that could precipitate is $\pu{01221 mol}$, because we would run out of carbonate ion Then there would be $\pu{00442 mol}$ of calcium ion left in $\pu{1 L}$ of solution for a concentration of $\pu{00442 M}$ Solubility product Calcium carbonate is not completely insoluble2010年3月24日 A series of experimental precipitated calcium carbonates (PCCs) coated with commercial stearic acid (stearin), with the coating amount of stearin added to the PCC particles ranging from 3 to 135 wt %, were prepared in aqueous medium and characterized by Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), and differential On the Coating of Precipitated Calcium Carbonate with Stearic What Is Calcium Carbonate Four Uses of Calcium Carbonate Calcium Carbonate Production Process Ground calcium carbonate is also called known as heavy calcium carbonate,it is made by mechanical methods (using grinding mills such as roller mill or other highpressure grinding mills) to directly crush natural calcite, limestone, chalk, shells, etc Ground calcium carbonate Calcium Carbonate – What Is It And How To Make It2023年9月28日 The material is fed into the grinding chamber between the grinding roller and the grinding ring, and the grinding roller applies pressure to the material, resulting in grinding and pulverizing of the calcium carbonate particlesThey are commonly used in the production of calcium carbonate powders with a fineness ranging from 80 mesh to 600 meshExploring Different Grinding Mills for Calcium Carbonate

Effects of Grinding Aids Used in Grinding Calcium Carbonate (CaCO3
2021年12月31日 Grinding aid chemicals which are used in the grinding of calcium carbonate (CaCO3) to prevent agglomeration are chemisorbed on the surfaces of particles, and the compatibility of them with the solvent, water, or organic resin affects the dispersion of the minerals and ultimately downstream product properties in consumer industries such as paint, Study with Quizlet and memorize flashcards containing terms like order the steps required to predict the volume (in mL) of 0100 M calcium chloride needed to produce 100 g of calcium carbonate There is an excess of sodium carbonate, calculate the volume (in mL) of 0100 M CaCl2 needed to produce 100 g of CaCO3(s), lab data and moreLab 9: Stoichiometry: Synthesis of Calcium Carbonate2024年5月21日 Contemporary models linking calcification to environmental conditions focus on parameters within the seawater carbonate system that are thought to operate as dominant drivers 4,5For example Multiple carbonate system parameters independently govern parameters pH, calcium, alkalinity, total dissolved solids, and temperature Then the computed Langelier index or calcium saturation index is used to gauge the aggressiveness of the water The tendency of a water to deposit a calcium carbonate (CaC03) coating is conceived as a form of protection against internal corrosion of metal pipes For aCalculating the pH of Calcium Carbonate Saturation JSTOR
.jpg)
How to Make Eggshell Calcium (and Why You’d Want to) Mama
2018年2月25日 Robert makes an excellent point here! Just try soaking a hard boiled egg in vinegar overnight This will extract all of the calcium from the shell (1 shell is about 2000mg calcium) The vinegar causes the carbonate to be separated from the calcium The carbonate evaporates as bubbles, and the calcium is left in the vinegarThe term "scale" is used to describe a hardened mineral buildup on surfaces, equipment and pipes of a water system Unfortunately, most definitions online refer only to calcium carbonate scale, also known as limescale As we will discuss later in this article, there are several other forms of scale that are not carbonate scale Here's our own definition of scale:Understanding Calcium Carbonate Scale Orenda Tech2022年1月12日 This article elucidates the effect of type of pigment/extenders viz titanium dioxide (TiO2), magnesium silicate (steatite), calcium carbonate, dolomite, precipitated sodium magnesium aluminosilicates, hydrated and calcined aluminum silicates (kaolin) when used alone or in combination in the paint dispersions on their sedimentation stability Accelerated heat age Investigation of effect of type of pigment/extender on the stability Two different industrial processes are used to manufacture calcium carbonate: the grinding of natural calcium carbonate The particle size distribution was analysed using the MalvernPanalytical Mastersizer 3000 laser diffraction system At the same time, Calcium carbonate: evaluating particle size and surface area
.jpg)
An Overview of Lime Slaking and Factors That Affect the Process
dry form or in flue gas desulphurization in slurry form The use of lime in carbonate form is beyond the scope of this paper We will concentrate on lime as calcium oxide and calcium hydroxide In most pollution control applications lime is used as calcium hydroxide To manufacture calcium hydroxide the limestone calcium carbonate must be 2023年6月19日 Calcium carbonate can be used as a calcium supplement: the absorption rate can reach 39%, second only to calcium fruit acid soluble in stomach acid, has become the most dosage form, the most used calcium supplement For grinding calcium carbonate, we recommend that you choose the HGM series ultrafine grinding mill Capacity: 05~45 t/hHow to Choose a Grinding Mill When Grinding Calcium Carbonate 2015年5月11日 $\ce{CaCO3}$ is basic so it will neutralize the acid $$\ce{CaCO3 + 2HCl > CaCl2 + H2O +CO2}$$ In your experiment you need to devise a way of finding out what the end point of the reaction is (using a $\mathrm{pH}$ indicator to find when the mixture is neutral) You need to be able to measure the amount of hydrochloric acid used in moles (you should How to calculate the percentage of calcium carbonate in H 2 CO 3, the rather weak carbonic acid, is important in controlling the solubility of carbonate minerals in most natural environmentsFor example, the decay of organic matter in a soil may produce CO 2 in large amounts The gas produced is readily soluble in water and a proportion combines with water to yield carbonic acid ()An increase in activity of the carbonic acid in Calcium carbonate and the carbonic acid system SpringerLink

(PDF) Calcium Carbonate Synthesis, Optimization
2020年7月30日 In this study, to determine the calcium carbonate availability in eggshells waste and factors those affect its extraction The parameters like temperature, the size of the eggshell powder and the assumption that passive scales were made up of iron hydroxide codeposited with calcium carbonate (we now know that it’s actually ferrous carbonate that forms) The LSI is based on the algebraic manipulation of the active ion product for calcium carbonate and that of the dissociation of the bicarbonate ion: 2+αCa 2 αCO 3 = Ksp (1) +αCO 3A NOVEL CALCIUM CARBONATE SCALING MODEL FOR 2018年1月1日 CO 2 hydrolyzes in water, making the bicarbonate and carbonate anions (discussed in more detail in section “CO 2Carbonic AcidCarbonate System and Seawater”) that react with divalent and monovalent metals 3 Among the divalent cations, calcium combined with the carbonate anion makes a solid of low solubilityCarbonate Minerals and the CO2Carbonic Acid System2023年6月25日 Crushing: The calcium carbonate stones just mined from the quarry are relatively large, and they need to be crushed by a jaw crusher and a hammer crusher in turn to the feed fineness (10mm20mm) that can enter the mill Grinding: Use a bucket elevator to send the crushed small pieces of calcium carbonate to the silo, then use a vibrating feeder to send them Guide to Calcium Carbonate Grinding: Mills, Tips, and Uses
.jpg)
Procedure for Calculating the Calcium Carbonate Precipitation
2020年12月28日 The calcium carbonate (CaCO3) precipitation potential (CCPP) can predict the potential for corrosion and lime scaling in drinking water systems CCPP can be calculated by different standards, but none of these consider all of the conditions in drinking water systems where temperatures can reach 100 °C and the water exchanges CO2 with the atmosphere 2002年3月11日 Grinding tests were carried out to determine the influence of optimum dispersant dosage on grinding mill performance All grinding tests were carried out in a Svedala 75 kW SAM (Sala Agitated Mill) mill; see Fig 1The SAM mill was developed for grinding middlings concentrates in mineral beneficiation processes as well as for fine or ultrafine grinding of A new method for determining the optimum dispersant concentration O system Calcium carbonate in water with a fixed partial pressure of carbon dioxide For the case of a fixed partial pressure of carbon dioxide and calcium carbonate dissolved in the aqueous phase one more equation is need to describe the system This is the solubility product of calcium carbonate: = K ( CaCO ) ( Ca ) ( CO ) o CaCO 3CARBONATE EQUILIBRIA UC Davis2019年7月22日 Recommend Calcium Carbonate Grinding Mill Capacity: 0430 T/H Feeding size: ≤20mm Output Size: 1503000mesh Calcium Carbonate Grinding Mill is the equipment specializing in producing fine and superfine calcium carbonate powderHow to make the calcium carbonate powder?
.jpg)
How To Calculate Alkalinity As Concentration Of CaCO3
2018年6月4日 4 Calculating Alkalinity as CaCO₃ To calculate alkalinity as calcium carbonate, use this formula to calculate the alkalinity in concentration of calcium carbonate (CaCO 3) Insert the values into the formula to determine the alkalinity as CaCO 3: Alk(mg/L as CaCO 3)=(50044xBxC a xCF)÷V s Inserting the example values, the equation becomes: